Abstract
Background: Venetoclax combined with azacitidine has been demonstrated to have a favorable overall response rate and tolerable safety in acute myeloid leukemia (AML) patients who are unfit for intensive chemotherapy.
Methods: This study retrospectively recruited 64 Adults (≥18 years) with newly diagnosed AML ineligible for intensive chemotherapy who had received at least one cycle of treatment with venetoclax plus azacitidine. The primary endpoint was overall survival (OS). Secondary endpoints were remission rates. Safety was evaluated based on the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
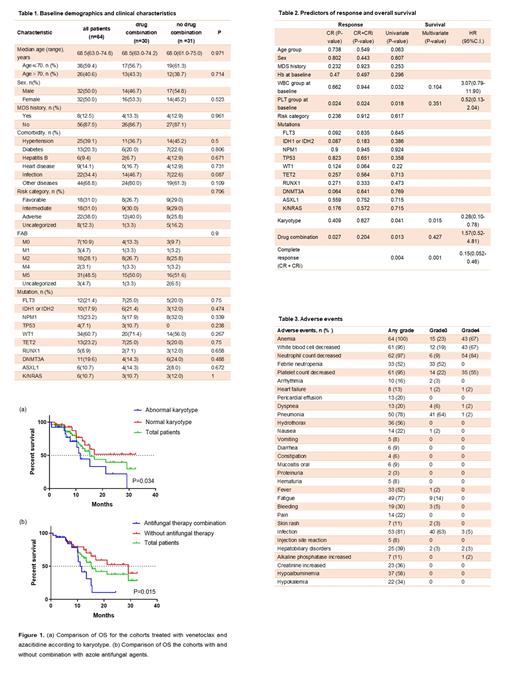
Results: The median age of the enrolled patients was 69 years. The median OS for the cohort was 15.5 months, with 21.2 months for complete responders as opposed to 13.3 months for those who did not achieve a complete response. 39 (60.9%) patients achieved composite complete remission (complete
remission (CR) + CR with incomplete count recovery (CRi)) and 7/64 (10.9%) achieved morphologic leukemia-free state (MLFS) with a median follow-up time of 14.7 months. The median CR+CRi duration was 10.9 months. It is noteworthy that 43/64 (67.2%) patients achieved the best response after one cycle with a median time of 1.2 months. Normal karyotype (P=0.015) was significantly associated with extended OS in multivariate analysis, while higher white blood cell count (P=0.032), lower platelet count (p=0.018) and antifungal drug combination (P=0.013) were predictors of shortened OS in univariate analysis. Common grade 3/4 Adverse Events (AEs) included infection (68%) and hematological AEs consisting of neutropenia (93%), anemia (90%), leukopenia (86%), thrombocytopenia (77%) and febrile neutropenia (52%). The median neutropenia duration was 16 days.
Conclusions: The novel venetoclax-azacitidine combination regimen showed prolonged survival period, promising efficacy, sound and rapid response, and superior tolerance in Chinese patients newly diagnosed AML.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal